The web Browser you are currently using is unsupported, and some features of this site may not work as intended. Please update to a modern browser such as Chrome, Firefox or Edge to experience all features Michigan.gov has to offer.
WHITING: A naturally occurring phenomenon

WHITING: A naturally occurring phenomenon
EGLE is often contacted by concerned citizens claiming that someone dumped a white milky substance into the lake. In some lakes, a naturally-occurring phenomenon makes the color of the water change from clear blue to gray or milky white.
This phenomenon is often the result of natural processes, not environmental pollution. In fact, the cause for this whiting phenomenon is the precipitation (coming out of the water as a solid) of calcium carbonate.
Calcium Carbonite
Calcium carbonate is a white, crystalline mineral that is widely distributed in nature and is the main ingredient in limestone, marble, coral, calcite, and chalk. Whiting events occur in lakes with very high concentrations of calcium carbonate (hard water lakes) during early summer. As the calcium carbonate precipitates, it forms chalky white clouds underwater and rains calcium carbonate on the lake bottom. When the calcium carbonate particles consolidate on the lake bottom, they form a soft rock called marl.
Depicted to the left: Marl from lake bottom and Calcite (Courtesy of Larry Bean, rock collector, Livonia, Michigan.)
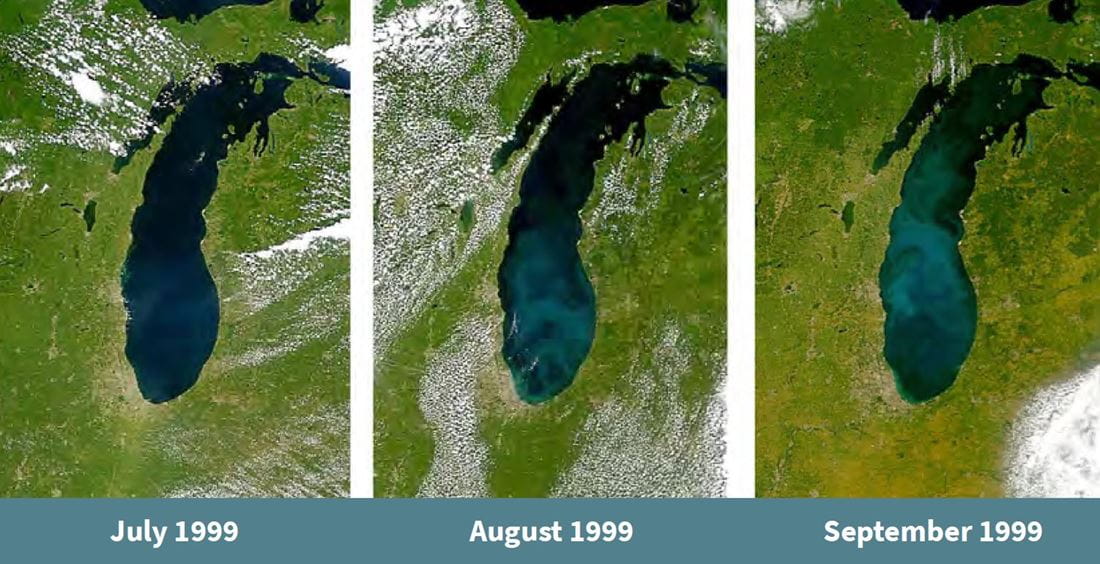
In the summers of 1998 and 1999, NASA’s satellite captured images of a mysterious flush of color that spread across Lake Michigan. The color change was attributed to either a whiting event or an algae bloom. Similarly, the title image depicts a whiting event occurring in Lake Erie in late April and early May 2002. (Image courtesy of Jacques Descloitres, MODIS Land Rapid Response Team at NASA GSFC)
Some white material in water can indicate pollution. When deciding if the milky appearance is natural or caused by pollution, consider the following:
- Proximity to a potential pollution source. Some industries such as mining, metal cutting, salt processing, and paper manufacturing have materials that can cause water to appear milky when released into the environment. A defined waste stream into the lake could indicate a pollutant source, while a sudden change of color from within the lake may indicate a whiting event.
- Time of year. Whiting events most often occur in early to mid-summer.
- Simple field test. Gather white particles by filtering some of the lake water through a fine filter. Next, place a drop of vinegar on the filtered white particles. Bubbling or fizzing will occur in the presence of calcium carbonate. This is the same reaction that would occur if you put vinegar on baking soda.
View/download a pdf of this brochure.
For more information, including tips to help reduce the amount of nutrients that can enter a lake from your home activities, contact any EGLE district office or call the State of Michigan’s Environmental Assistance Center at EGLE-Assist@Michigan.gov or 800-662-9278.
If you find pollution and believe it is human-induced, please report it to the State of Michigan’s Pollution Emergency Alerting System (PEAS) hotline at (800) 292-4706.